An ideal gas is a theoretical idea – a gas in which there are no attractive forces between the molecules, and in which the molecules take up no space. Both of these assumptions are incorrect, for:
However:
Under these conditions, a gas will behave nearly ideally. This is true for things we readily identify as gases at room temperature and pressure conditions, like hydrogen, nitrogen and oxygen. These real gases are said to behave ideally – that is, they obey the ideal gas law.
The ideal gas law is usually stated as pV = nRT, where
of the gas, and
As long as the pressure is not too high, and the temperature is fairly warm, this law is followed very closely by most real gases.
The value of R is determined experimentally by measuring the other variables in the equation, and solving mathematically to get the value of the constant. R is the same for all gases – provided the gas has ideal behavior.
In this experiment you will determine the ideal gas constant using H2 gas. The H2 will be generated using this reaction:
Mg (s)+ 2 HCl (aq) ![]() MgCl2 (aq) + H2
(g)
MgCl2 (aq) + H2
(g)
From the balanced equation, you can see that there is a simple ratio between the number of molecules of Mg used and the amount of H2 produced. By measuring the mass of Mg used we can calculate the number of moles of Mg produced, and thus determine n for the ideal gas equation. T can be measured with a thermometer, p from a barometer, and V will be measured in a special gas buret. With these four pieces of information, you'll be able to calculate R.
Materials:
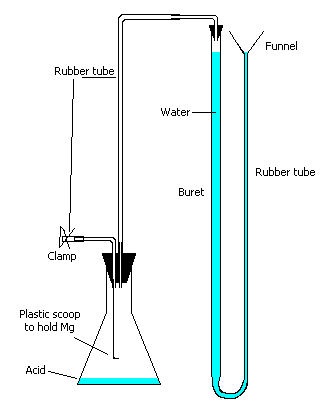
gas buret apparatus (as shown in diagram – see teacher notes)
ring stand
clamp
1.0 M HCl
thermometer
magnesium metal ribbon
Procedures:
Wear goggles at all times during this experiment.
 1. Cut a piece of magnesium
ribbon no longer than the length suggested by your teacher. Make sure the ends are square.
Find the mass of the ribbon. Measure the length of the ribbon after cutting it, and divide
it as exactly as possible into three equal pieces. Caution: make sure not to use more
ribbon than suggested. If you do so, you will overfill the gas apparatus, and will not be
able to measure the results.
1. Cut a piece of magnesium
ribbon no longer than the length suggested by your teacher. Make sure the ends are square.
Find the mass of the ribbon. Measure the length of the ribbon after cutting it, and divide
it as exactly as possible into three equal pieces. Caution: make sure not to use more
ribbon than suggested. If you do so, you will overfill the gas apparatus, and will not be
able to measure the results.
2. Set up the apparatus as shown in the diagram, but leave the stoppers out of the flask and buret at this time. Clamp the buret, and funnel near the top of the ring stand. Fill the buret with water until the level of water is at the 0 mark on the buret, and just at the bottom of the funnel, as shown in the diagram. Then insert the stopper into the top of the buret.
3. Pour about 40 to 50 mL of 1 M HCl into the flask. You need not measure it accurately – just use the calibration marks on the flask.
4. Put one of the pieces of Mg metal into the plastic scoop (the scoop is made from the cut off end of a Berol pipet). Make sure that it won't fall out into the acid too easily, but that you will be able to shake it loose when you want to.
5. Put all the stoppers tightly in place. With the clamp off the tube, adjust the level of the buret until the water is at the 0.0 mL mark on the buret. Now close the clamp.
| Your apparatus
must not leak. To test for leaks lower the funnel part way. At first the water level will
change but if the apparatus is gas tight the water level will stop rising in the funnel
and remain constant. You can even lower the funnel below the flask level if the setup is
gas tight. If the apparatus is leaking the water level will continue to change. If it does leak fix it before going on. |
6. Raise the funnel back to the top, and shake the Mg metal into the acid. It will begin to react quickly and produce H2 gas.
7. When all sign of any reaction stops, wait about five minutes for the apparatus to come to room temperature. Measure the room temperature at this time.
 8.
Adjust the height of the funnel so that the level of water in the buret and the funnel are
the same. When the heights are the same, the gas pressure inside the tube is the same as
the atmospheric pressure in the room. Now read the gas volume from the water level in the
buret.
8.
Adjust the height of the funnel so that the level of water in the buret and the funnel are
the same. When the heights are the same, the gas pressure inside the tube is the same as
the atmospheric pressure in the room. Now read the gas volume from the water level in the
buret.
9. Repeat steps 4 to 8 for the other two pieces of Mg. You should not need to change the acid. Make sure that the plastic bowl is dry before beginning each trial.
10. Measure the atmospheric pressure using a barometer.
11. Clean up the apparatus. Wash everything with water.
Calculations and Conclusion:
1. Since you divided the Mg into three equal pieces, calculate the mass of 1 piece and then from the molar mass of Mg, the number of moles of Mg used. Using the balanced equation, indicate how many moles of H2 gas will be produced. This is n for use in the ideal gas equation.
2. Calculate the temperature in K. If your barometer does not measure in kPa, you will have to convert its reading into the proper units. For example if it measures in mm Hg, then this relationship can be used to do the conversion:
760 mm Hg = 101.3 kPa
For example, if the room pressure was 700 mm, you could use this formula as:
| (700 mm Hg)(101.3 kPa) | = | x kPa |
760 mm Hg |
3. The gas in the tube is mostly H2. However, there is also some water vapor in the tube. The amount of water vapor depends only on the temperature of the water (assumed to be room temperature) and can be found from the following table (the vapor pressure of water in kPa):
| Temperature (oC) | Pressure (kPa) | Temperature (oC) | Pressure (kPa) | |
| 15 | 1.71 | 23 | 2.80 | |
| 16 | 1.81 | 24 | 2.99 | |
| 17 | 1.93 | 25 | 3.16 | |
| 18 | 2.06 | 26 | 3.36 | |
| 19 | 2.20 | 27 | 3.59 | |
| 20 | 2.33 | 28 | 3.77 | |
| 21 | 2.48 | 29 | 4.00 | |
| 22 | 2.64 | 30 | 4.24 |
From the above table, determine the amount of water vapor in the buret at the temperature used for the experiment. The total pressure in the buret is equal to the room pressure, but it is made of two parts:
This can be expressed using Dalton’s law of partial pressures as:
proom = pHydrogen +pwater vapor
You measured the proom with the pressure gauge. Use the equation to calculate the pHydrogen in the buret. This will be the value of p to use in the next step.
4. Change the volume from mL into L (doing so will make it easier to compare the value of R to the accepted value in the next step). Substitute the known values for p, V, n and T into the ideal gas equation, and solve it for the value of R. What is the unit for R?
5. Repeat step 4 for your other two trials, and calculate the mean value of R. Research in a book to find out the accepted value of R. Calculate your error from this accepted value, and express it as a percentage error.
Copyright © 1998 - 2008 David Dice