 Figure 1
Figure 1David Dice
Feb 10, 2001
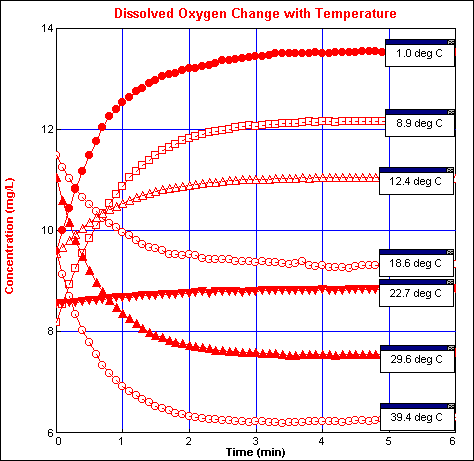
It is not simple to get accurate readings of dissolved oxygen using the Vernier dissolved oxygen probe when the temperature is changing. Factors that must be considered include the amount of time required to saturate/change the saturation of oxygen dissolved in the water. However, the primary issue seems to be the amount of time it takes to temperature stabilize the probe. If the temperature is changing, then at least 4 minutes per measurement should be allowed in order for the probe to give proper readings of dissolved oxygen. When proper temperature equilibration is allowed, then the readings measured are accurate within the stated reproducibility of the probe (± 0.2 mg/L).
200 mL of deionized water was placed in a 2 L pop bottle. The bottle was capped, then the temperature of the water was varied by running the bottle under either hot or cold water, while shaking intensely. The bottle was opened periodically to allow the air pressure to equilibrate. The bottle was finally shaken violently for 1 minute, and then the water was poured into a styrofoam cup where it was stirred vigorously with a magnetic stirrer. In cases where the water temperature was near 0 oC, deionized water ice was added to the water to lower its temperature. Small amounts of ice were present in the water in the lowest temperature trials. Atmospheric pressure was 729 Torr, and room temperature was 22 oC during the trials.
The Vernier oxygen sensor and stainless steel temperature probe were lowered into the cup containing the water to the same depth in each trial. The resulting measured oxygen content was recorded for 6 minutes using the Lab Pro interface and Logger Pro software.
 Figure 1
Figure 1
It takes a significant length of time for the probe to stabilize. As shown in figure 1, the data changes significantly for at least 3 minutes, and approximately 4 minutes is required for the probe to become stable. When the temperature of the water samples is changing, the probe must be left in the water sample for about 4 minutes in order for the temperature to stabilize. Figure 2 shows the type of results that can be expected when following the above procedure. It is important to note that consistent readings can be obtained with the Vernier dissolved oxygen probe when care is taken to ensure that the water is truly saturated with oxygen, and sufficient time is allowed to stabilize the temperature compensation of the probe. If this is done, then the probe gives results consistent with its intended use as a teaching tool in indroductory chemistry and biology.
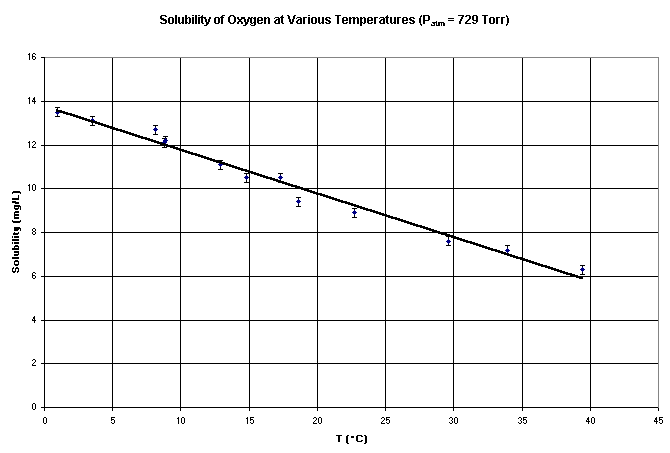 Figure 2
Figure 2