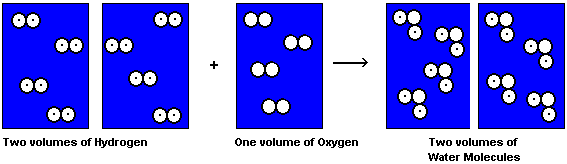Avogadro's HypothesisScience creates knowledge using two processes:
Occasionally both processes are carried out by the same person; often however, the observations have occurred long before a satisfactory explanation for them is thought up. An hypothesis is a tentative (as opposed to a theory which is well tested) explanation for observed events. An hypothesis is not a prediction, but it must allow you to make predictions which can be tested by experiment. When the results of those experiments are as predicted, it lends support to the hypothesis as a good explanation, and its eventual acceptance as a theory. If the results are not as predicted, the hypothesis must be modified, or replaced with a better explanation. No statement is an hypothesis unless it suggests a cause for an effect, and unless it has the possibility of being wrong.
Truly revolutionary hypotheses, the kind that invalidate established beliefs, have often been initially rejected. This is especially true when a new idea contradicts that of well known scientists. Even scientists whose ideas were initially rejected accept this as the way science should work. For science is also full of examples of revolutionary ideas that did not stand up under the scrutiny of testing and verification. The non-discovery of cold fusion is probably one of the best known examples of this process in recent scientific history. Skepticism is one of the scientist's main tools.
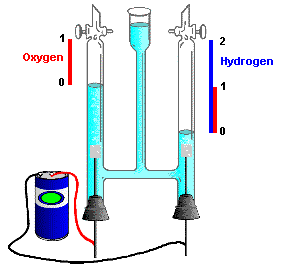
This observation - simple whole number combining ratios by volume - became known as the "Law of combining volumes". These results certainly suggested that the formulas for water might be H2O, for ammonia NH3, and for Nitrogen dioxide NO2.
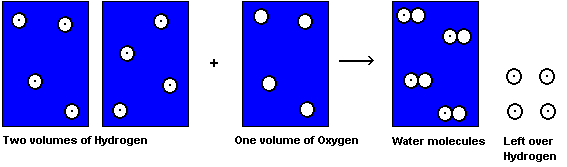
However, this observation was not accepted by everyone. John Dalton for one, considered
Gay-Lussac's work wrong. He believed water contained just one atom of hydrogen and
one atom of oxygen (
Since we don't think water is HO today, Dalton's idea must have been all wet! His conclusion that Gay-Lussac was wrong was not agreed to by everyone, including an Italian chemist, Amedeo Avogadro.
The reaction between Hydrogen and Oxygen according to Avogadro is shown in the following diagram. Notice that each container has the same number of molecules in it. The ratio of the volumes is 2 hydrogen to 1 oxygen to 2 water, and there is nothing left over. This proposal, published in the French Journal de Physique went neglected by the European scientific community. Partly because it was published in an obscure journal, partly because it totally contradicted the opinions of John Dalton, it took fifty years before Avogadro's work was recognized. Avogadro made the distinction between atoms and molecules, which today seems clear. However, Dalton rejected Avogadro's hypothesis because Dalton believed that atoms of the same kind could not combine. Since it was believed that atoms were held together by an electrical force, only unlike atoms would be attracted together, and like atoms should repel. Therefore it seemed impossible for a molecule of oxygen, O2, to exist. Avogadro's work, even if it was read appears not to have been understood, and was pushed into the dark recesses of chemistry libraries and ignored. Avogadro continued to teach at the university of Turin, when it was not closed because of the political upheavals going on in Italy at the time, and died in 1854, an unknown figure.
Without a clear distinction between atoms and molecules, European chemistry blundered ahead. By 1860 enough data had been collected on atomic weights to clearly indicate that something was seriously wrong. The world's first international conference on Chemistry was called at Karlsruhe, Germany in 1860 to try and resolve the issues. At this meeting, Stanislao Cannizaro, Avogadro's countryman, younger by fifty years, made a strong speech in which he explained the ideas of Avogadro that he had discovered two years earlier. He distributed copies of his "Sketch of a Course of Chemical Philosophy" which clearly showed the importance of Avogadro's principle, resolving the confusion with atomic and molecular weights. Very shortly, Avogadro's work was recognized for its brilliant deduction. With a clear understanding of how to measure atomic and molecular weights established, Chemistry proceeded rapidly. Less than a decade later, Dmitri Mendeleev who had also attended the Karlsruhe conference was able to use the newly established atomic weights to develop his periodic table. While chemistry is generally agreed to have started with Lavoisier, it could well be argued that modern chemistry started after the Karlruhe conference and the acceptance of Avogadro's hypothesis. In recognition of his idea, the number of fundamental particles in a mole of substance is called Avogadro's number. Although Avogadro had no idea what the number of particles in equal volumes of gases was, and did nothing to measure it, his hypothesis did lead to the eventual determination of this number, 6.02 x 1023 Copyright © 1998 - 2008 David Dice |
||||||||||||||||||||||||||||||||||||||||||||||

 Lorenzo Romano Amedeo Carlo Avogadro di Quareqa e di
Carreto - Avogadro for short - was born in Turin, Italy in 1776, into a family of church
lawyers. He too studied ecclesiastical law, earning his Bachelor's degree at the age of
16, and a Doctorate by 20. After three years he decided that a career in law was not to
his bent, and he began to study science and mathematics in earnest. In 1809 he was
appointed professor of Natural Philosopy at the Royal College of Vercelli in isolated
Northern Italy. In 1811 he proposed his now famous hypothesis that equal volumes of
gases, at the same temperature and pressure, contain equal numbers of molecules.
Lorenzo Romano Amedeo Carlo Avogadro di Quareqa e di
Carreto - Avogadro for short - was born in Turin, Italy in 1776, into a family of church
lawyers. He too studied ecclesiastical law, earning his Bachelor's degree at the age of
16, and a Doctorate by 20. After three years he decided that a career in law was not to
his bent, and he began to study science and mathematics in earnest. In 1809 he was
appointed professor of Natural Philosopy at the Royal College of Vercelli in isolated
Northern Italy. In 1811 he proposed his now famous hypothesis that equal volumes of
gases, at the same temperature and pressure, contain equal numbers of molecules.