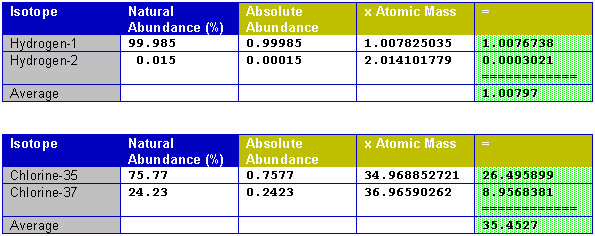
IsotopesMost atoms have several naturally occurring isotopes (click here for a list of elements that have no isotopes). An isotope is an atom which contains a different number of neutrons in its nucleus than some other atom of the same element. This means that different isotopes of an element will have different masses, since both the protons and the neutrons contribute about equally to the mass of an atom. (Here is a great source of information about sub-atomic particles from a physics point of view.) Not all isotopes are equally abundant in nature. For example, here are the naturally occurring isotopes of Hydrogen (Hydrogen-2 is the only common isotope which has its own name, and is generally called Deuterium).
Compare these abundances of hydrogen to those for some other elements. Notice that the atomic mass is close to the sum of the number of neutrons and protons, but not exactly the same.
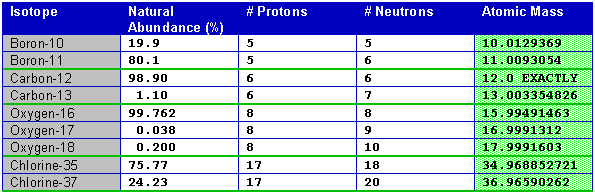
Carbon-12 is special, since its mass is defined to be exactly 12. It is the element chosen to be the reference mass in calculating atomic masses. You'll also notice that the atomic masses of each element are known very accurately. It would be impossible to construct a balance that had moving parts with that kind of accuracy--the vibration of the earth's crust would never allow it to become stable. Today's atomic masses are not calculated using a balance at all. Instead, they are measured using various forms of mass spectroscopy. A mass spectrometer can provide extremely accurate measurement of the mass of an individual atom. Here's how it works. A moving charged particle (an ion of an atom) will bend if it is placed in a magnetic field. The amount that it bends depends on its charge (+2 charged particles would bend twice as much as +1 charged ones, and negatively charged particles would bend the opposite direction). More importantly, the amount that the particle's path curves depends on its mass. It is sort of like rolling a steel shot put past a magnet. Because of its heavy mass, the path of the shot put would be very straight, compared to the path for a small ball bearing. In other words, a light particle will bend more in a magnetic field. In a mass spectrometer, atoms are charged by bombarding them with electrons. These charged atoms pass through a strong magnetic field, and their path bends. The lightest atoms bend a lot. The heavier atoms bend very little. Some type of detector is used to measure where the atoms arrive. By measuring the curvature of the path, and comparing it a known atom - our standard being Carbon-12 - very accurate masses can be calculated.
Once the precise atomic masses of each isotope, and their relative abundances are known, the average mass of the isotope as found in nature can be calculated. In fact, the atomic masses are known very precisely, as shown in the above tables, but the abundances are much less accurately known. This is partly because the abundance of isotopes can vary from one location on earth to another. Certain naturally occurring process can concentrate the abundance of one type of isotope in one location as compared to another. Lead's isotopic abundance is one of the least reproducible because various isotopes are the final products of the radioactive decay of a number of heavy elements. To find the average mass of the mixture of isotopes found in nature the following procedure is used: multiply the abundance of each naturally occurring isotope of element by its precise atomic mass and then sum them all. Here are the results for a couple of elements.
Copyright © 1998 - 2008 David Dice |

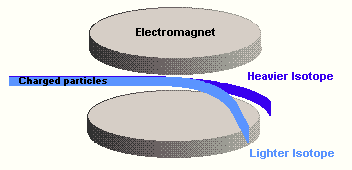 In the original mass
spectrograph, a photographic film was the detector. The film would be darkened by the
impacting charged particles. The more particles that arrived, the darker the film would
become. Measuring the amount of this exposure allowed scientists to calculate the
percentage abundance of each isotope. In a modern mass spectrometer electronic devices are
used to measure the number of ions which arrive, but the principle remains the same -
heavy atoms and light atoms can be separated very precisely on the basis of their
mass.
In the original mass
spectrograph, a photographic film was the detector. The film would be darkened by the
impacting charged particles. The more particles that arrived, the darker the film would
become. Measuring the amount of this exposure allowed scientists to calculate the
percentage abundance of each isotope. In a modern mass spectrometer electronic devices are
used to measure the number of ions which arrive, but the principle remains the same -
heavy atoms and light atoms can be separated very precisely on the basis of their
mass.