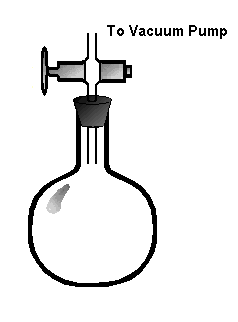The Relative Masses of Gases
CAUTION!
This demonstration uses equipment under vacuum.
Because of the danger of implosion it must be carried out only by those properly trained
in handling evacuated glass containers.
|
A safety shield must be in place at all times, and hand
and eye protection must be worn.
It must be carried out as a demonstration, not as an experiment conducted by students. |
Materials:
- 500 mL round bottom flask
- one hole rubber stopper
- vacuum stopcock
- vacuum pump
- source of gases such as oxygen, carbon dioxide and methane (you can use natural gas as a
source of relatively pure methane gas)
- balance or scale capable of measuring the mass of the assembled flask and stopper to the
nearest 0.01 g
Procedures: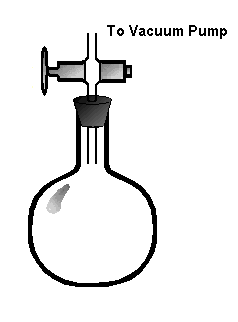
- Carefully insert the stopcock into the rubber stopper, and place the stopper into the
flask as shown in the diagram.
- Attach to a vacuum pump with a length of rubber tubing, and evacuate the flask. Close
the stopcock and disconnect the rubber tubing. Find the mass of the empty flask. Use
extreme caution not to bump the flask against any hard object while it is evacuated.
- Connect the closed flask to a source of dry compressed gas. Using the regulator on the
compressed gas, fill the flask to just slightly above atmospheric pressure, and then
disconnect the flask. Leave the stopcock on the flask open, so that the pressure inside
the flask drops to atmospheric, and find the mass. Use caution not to overfill the flask,
or the pressure will blow out the stopper.
- Repeat steps 2 and 3 for each available compressed gas.
- Complete the calculations necessary to fill out the following data table. Since the
flask is always at the same temperature and pressure, it should always have the same
number of molecules of each gas in it. This allows us to calculate the relative mass of
each gas compared to a standard. In this case the standard selected is Oxygen at a molar
mass of 32. If we multiply this value by the relative mass, we will have an experimental
molar mass for each gas.
| Gas Used |
Mass of Flask
Assembly
and Gas (g) |
Mass of Empty
Flask Assembly
(g) |
Mass of Gas
in Flask
(g) |
Relative Mass
Compared
to O2 |
Experimental
Molar Mass |
| Oxygen |
? |
? |
? |
1.0 |
32 |
| Carbon Dioxide |
? |
? |
? |
? |
? |
| Methane |
? |
? |
? |
? |
? |
Discussion:
- Are the conditions for Avogadro's hypothesis being followed closely enough in this
experiment that we can assume that there are the same number of molecules in the container
each time?
- Why does assuming there are the same number of molecules in the container each time
allow you to calculate the molar mass?
- What are some of the main sources of systematic error in this experiment?
Alternative Procedure:
If you do not have a vacuum stopcock, then you can improvise one using a length of
glass tubing, and a short (10 cm) piece of light rubber tubing (do not use thick wall
vacuum tubing). Put the glass tubing through the stopper, and attach the rubber tubing to
the other end. Attach the vacuum pump to this rubber tubing (you will need a second length
of glass tubing to join the hose from the vacuum pump to this flexible tubing). When the
flask has been evacuated, pinch the flexible tubing into a tight bend, and clamp it off
with a pinch clamp.

Copyright © 1998 - 2008 David Dice |