Kelvin temperature: the absolute
temperature scale, which must be used in all To calculate the Kelvin temperature, add
273.15 to the Celsius temperature. Click here for help on converting temperature scales.
Kinetic energy: energy that an object
possesses because of its motion. Kinetic energy depends on the temperature of a substance. The energy in a molecule is
distributed amongst its rotations, translations and vibrations.


|
- Rotation is the movement of a molecule about an axis.
- Translation is the movement of a molecule from one place to another.
- Vibration is the motion of one atom in relation to another within a molecule. In a water
molecule there are in-and-out and wig-wag vibrations. Vibration is the only motion allowed
for molecules in the solid state.
|
In a real molecule all of these motions are combined at once. As the temperature
increases, these movements will also increase, following a Maxwell-Boltzmann distribution.
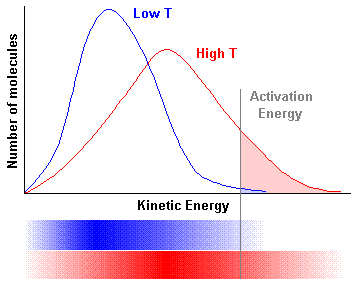 |
At any given temperature, the molecules in a sample of gas travel at a
variety of speeds. The top portion of this graph shows the standard way of representing
a Maxwell-Boltzmann distribution. At a low temperature the distribution of energies is
lower. As the temperature increases, there are more molecules with higher energies. The
number of molecules which can react – those with kinetic energies greater than the
activation energy – are represented by the shaded area to the right of the activation
energy.
The lower portion of the graph shows a comparison to the bar graph format used in the potential energy video. |
Kinetic molecular theory:
first proposed in the 1860's independently by Boltzmann and Maxwell, this theory explains
the behavior of an ideal gas using the four following ideas:
- a gas is made of a very large number of molecules so small compared to
the distances between them, that their size can be ignored (dimensionless particles).
- the molecules are in constant, rapid, straight line motion, colliding
frequently with each other and the walls of any container in which they are placed. These
collisions are perfectly elastic, that is, no kinetic energy
is lost or gained during collision.
- the molecules do not exert any attractive or repulsive force on each
other.
- although there is a wide distribution of kinetic energies, the average
kinetic energy is proportional to the Kelvin temperature.
