 Experimental setup for measuring the approach to equilibrium for a saturated solution of CuSO4 in water.
|
 Experimental setup for measuring the approach to equilibrium for a saturated solution of CuSO4 in water.
|
When an ionic solid such as CuSO4 dissolves in water, it dissociates into ions, as represented by this equation:
CuSO4 (s) ![]() Cu2+ (aq) + SO42- (aq)
Cu2+ (aq) + SO42- (aq)
Solutions of copper sulfate are blue, so the change in concentration of the ions can be detected visually -- as the concentration of the ions increases the solution becomes more blue. When an unchanging color is reached, the solution has reached equilibrium. The electrical conductivity of the ions can be measured at the same time. As the amount of ions in solution increases, so does the conductivity of the solution.
In this time lapse video, copper sulfate is dissolved in water1.
At first, the color changes rapidly as the solid copper sulfate dissolves. Initially
only the forward reaction takes place. However, as the concentration of the ions
begins to increase, the reverse reaction starts to increase in speed. The result is
that the net rate of solution drops. This can be observed as the
color change is very rapid at the start of the reaction, but it soon slows down.
The electrical conductivity shows the same effect.
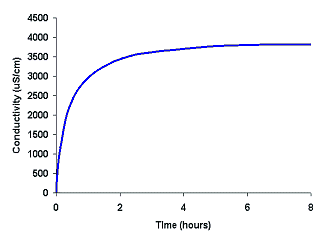 As the reverse rate becomes faster2,
the net reaction becomes very slow, so that the color and conductivity no longer change
much. It takes several hours for the solution to reach an equilibrium3, but as shown in this graph, after about 6 hours there is almost no
change in the conductivity of the solution. At this point, since the observable
properties are no longer changing, the reaction has reached equilibrium.
As the reverse rate becomes faster2,
the net reaction becomes very slow, so that the color and conductivity no longer change
much. It takes several hours for the solution to reach an equilibrium3, but as shown in this graph, after about 6 hours there is almost no
change in the conductivity of the solution. At this point, since the observable
properties are no longer changing, the reaction has reached equilibrium.
I2(s) ![]() I2(aq)
I2(aq)
3 I2(aq) + 3 H2O(l) ![]() IO3-(aq) + 5
I-(aq) + 6H+(aq)
IO3-(aq) + 5
I-(aq) + 6H+(aq)
I2(aq) + I-(aq) ![]() I3-(aq)
I3-(aq)
 However, the net result is the same, as there is an initial rapid rise in
color, followed by a gradual leveling off and reaching a constant fixed color. The
conductivity, though much lower than for an ionic substance like copper sulfate, shows the
same behavior.
However, the net result is the same, as there is an initial rapid rise in
color, followed by a gradual leveling off and reaching a constant fixed color. The
conductivity, though much lower than for an ionic substance like copper sulfate, shows the
same behavior.
1The solubility curve was measured by adding a fixed quantity of solid copper sulfate (CuSO4.5H2O) to 100 mL of solvent. The crystals were approximately 4 to 5 mm in size (this size was selected to allow a reasonable time for equilibrium to be achieved). The system was stirred at a constant rate, using a magnetic stirrer. The electrical conductivity, in units of µS/cm, was measured using a Vernier conductivity probe attached to a CBL/TI-82 calculator. In order to keep the conductivity at a measurable level, the solvent used was a 25% (v/v) solution of isopropanol in water. This decreases the solubility of the copper sulfate somewhat from its value in pure water.
2The amount of solid present in the system was large enough that there should be only a minimal effect on the rate of the forward reaction because of a decrease in reactants. Since very little of the solid dissolves, the surface area does not change very much. Therefore, the forward reaction rate will not slow down with time. In this case, the reason the two rates become equal is because of the increase in the reverse rate, rather than any decrease in the forward rate.
3The time required to reach equilibrium will depend on the rate of stirring, the temperature, and the surface area of the solid copper sulfate.