Remember that an equilibrium requires equal forward and reverse rates. You cannot have a rate in the forward direction if you have no reactants. You cannot have a rate in the reverse direction if you have no products. Therefore, equilibrium can only exist when you have some of both the reactants and products in the same system.
The rate of a reaction depends on:
Suppose that for the imaginary, simple reaction R ![]() P the rates were
P the rates were
Equilibrium occurs when the forward rate equals the reverse rate so:
There are two reasons why this equality may not be reached.
Case 1: If the rate constant of the forward reaction is very large compared to that of the reverse reaction, the concentration of reactant molecules must almost become zero before the two rates can become equal. This can be shown graphically as:
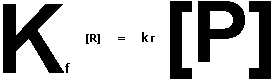
 Because Kf is very big
compared to Kr, [R] will have to become very small, and [P] very large in order
for both sides to be equal. Such a reaction is said to go to completion, since there
is virtually no reactant left.
Because Kf is very big
compared to Kr, [R] will have to become very small, and [P] very large in order
for both sides to be equal. Such a reaction is said to go to completion, since there
is virtually no reactant left.
A reaction like this is the combustion of methane:
CH4 (g) + 2 O2 (g) ![]() CO2 (g) + 2 H2O
(g)
CO2 (g) + 2 H2O
(g)
At high temperatures, the reaction in the forward direction (CH4 + 2 O2 burning to make carbon dioxide and water) is many, many times faster than the reverse reaction (CO2 + 2 H2O reacting to create methane and oxygen). Therefore, we normally consider this reaction to go to completion, and don't treat it as an equilibrium – though it may well be a steady state.
Case 2: A reaction will go to completion if the amount of reactants is so small there is not enough to reach the equilibrium concentration of products. For example, at room temperature (25 șC), the concentration of water vapor at equilibrium is 1.27 x 10-3 M. This means that if you put less than 1.27 x 10-3 moles of liquid water into a 1 L container, all the water will evaporate. There just isn't enough water to reach an equilibrium – the reverse rate will never catch up to the forward rate.
Another example of the same principle is a saturated solution. At saturation, NaCl has a solubility of about 360 g/L at room temperature. If you put less than 360 g of salt into a litre of water, all the salt will dissolve. An un-saturated solution is not an equilibrium.