Changing Equilibrium: Temperature
We already know that changing temperature will change an equilibrium. This was
illustrated in the simulation of the hydrogen iodide
equilibrium.
Here's why:
The rate of a chemical reaction depends on the temperature. In order for
molecules to react they must collide, and the kinetic energy of the collision must
be great enough to change the molecule's potential energy and allow the
atoms to rearrange into new substances. The potential energy holding the atoms to
each other in the original molecule is a potential energy barrier to change, and is called
the activation
energy (Ea).
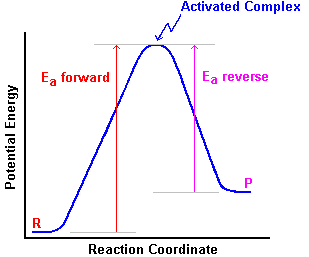 The reaction rate depends on the temperature
because, with a higher temperature, more molecules will be travelling faster. More
will have enough kinetic energy to make it over the activation (potential) energy barrier
as they collide. Notice, however, that as shown in the diagram, the
activation energy in the forward and reverse directions are not the same.
Because of this, the reaction rates in the forward and reverse directions will not
be affected equally by changing temperature.
The reaction rate depends on the temperature
because, with a higher temperature, more molecules will be travelling faster. More
will have enough kinetic energy to make it over the activation (potential) energy barrier
as they collide. Notice, however, that as shown in the diagram, the
activation energy in the forward and reverse directions are not the same.
Because of this, the reaction rates in the forward and reverse directions will not
be affected equally by changing temperature.

Click to see a video explanation.
|
 |
 |
 |
When the activation energy in the forward direction is larger (as shown here for an
endothermic reaction), an increase in temperature will make the forward reaction increase
more rapidly than the reverse. So, for a while, the rate will go faster in the
forward direction. This is no longer an equilibrium,
since the rates aren't equal any longer. Of course, as the reaction goes forward
faster it will make more products, P. More P will increase the rate in the reverse
direction. Eventually, we'll get back to an equilibrium, but it won't be the same
one as we started with. Temperature change has caused a change to a new equilibrium.
Exactly the opposite would happen if the reaction was exothermic, so that the
activation energy in the reverse direction would be greater. A change in temperature
would result in the rate initially going faster in the reverse direction until enough
products had decomposed that the rate would slow down to the point where the forward and
reverse rates were once again equal. This would once again be a new equilibrium.
So, in general, we expect a change in temperature to have an effect on an equilibrium.
 The reaction rate depends on the temperature
because, with a higher temperature, more molecules will be travelling faster. More
will have enough kinetic energy to make it over the activation (potential) energy barrier
as they collide. Notice, however, that as shown in the diagram, the
activation energy in the forward and reverse directions are not the same.
Because of this, the reaction rates in the forward and reverse directions will not
be affected equally by changing temperature.
The reaction rate depends on the temperature
because, with a higher temperature, more molecules will be travelling faster. More
will have enough kinetic energy to make it over the activation (potential) energy barrier
as they collide. Notice, however, that as shown in the diagram, the
activation energy in the forward and reverse directions are not the same.
Because of this, the reaction rates in the forward and reverse directions will not
be affected equally by changing temperature.