|
|
|
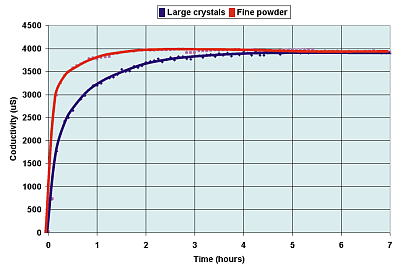
 The crushed copper sulfate crystals on the
left have a much greater surface area than do the larger crystals on the right.
Though any one small crystal has a smaller surface, there many more of them.
Therefore, the surface area of the crushed sulfate is greater. |
Changing Equilibrium:
Surface area
To a first
approximation changing the surface area has no effect on the final equilibrium
condition. If the surface
area is larger, then a reaction will reach an equilibrium more quickly, but the final
position reached is the same as with a smaller surface area.
Here's why:
Increasing the surface area in a heterogeneous reaction will normally increase the reaction rate. It does so because with
more surface area exposed between the two reacting states, there will be more collisions
and therefore a greater chance for a reaction.
For example, if we crush a solid before trying to dissolve it in water, it will have
more surfaces exposed. Since it can only dissolve from the outside edge, the larger
number of surfaces will dissolve more quickly. At the same time, with more surfaces
exposed there will also be more sites available for the molecules in the dissolved state
to re-crystallize.
Similarly, a large beaker of water has more surface area than a small beaker. The
water can evaporate faster from a larger surface. However, the water can also
condense faster from the vapor state, since there is more surface area available for the
reverse reaction as well.
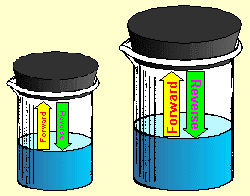 Because of this, the reaction rates in the forward
and reverse directions will be affected equally by changing surface area. The
reaction will go faster in the forward direction because of an increase in surface
area. It will go just as much faster in the reverse direction. Since both
forward and reverse reaction rates are effected the same, there is no net change to the
equilibrium once it has been achieved. The net result is that surface area has no
effect on vapor
pressure equilibrium. The vapor pressure of a pure liquid depends only on its
temperature.
Because of this, the reaction rates in the forward
and reverse directions will be affected equally by changing surface area. The
reaction will go faster in the forward direction because of an increase in surface
area. It will go just as much faster in the reverse direction. Since both
forward and reverse reaction rates are effected the same, there is no net change to the
equilibrium once it has been achieved. The net result is that surface area has no
effect on vapor
pressure equilibrium. The vapor pressure of a pure liquid depends only on its
temperature.
What about before you reach equilibrium? Then the rates are not equal.
Increasing the surface area means that you get to equilibrium more quickly.
However, eventually you reach a point where the rates in the forward and reverse
directions are equal.
The approach to equilibrium is different, but the final equilibrium achieved is the
same with and without the change in surface area.
So, in general, we expect surface area to have no effect on
an equilibrium, once equilibrium is established.

 Because of this, the reaction rates in the forward
and reverse directions will be affected equally by changing surface area. The
reaction will go faster in the forward direction because of an increase in surface
area. It will go just as much faster in the reverse direction. Since both
forward and reverse reaction rates are effected the same, there is no net change to the
equilibrium once it has been achieved. The net result is that surface area has no
effect on
Because of this, the reaction rates in the forward
and reverse directions will be affected equally by changing surface area. The
reaction will go faster in the forward direction because of an increase in surface
area. It will go just as much faster in the reverse direction. Since both
forward and reverse reaction rates are effected the same, there is no net change to the
equilibrium once it has been achieved. The net result is that surface area has no
effect on