Manufacturing Nitrates: the Ostwald process
Once ammonia has been produced by the Haber process, it can be converted into nitric
acid through a multi-step procedure known as the Ostwald process. In the first step
in this reaction, ammonia and oxygen gas catalytically react to form nitrogen monoxide:
4NH3(g) + 5O2(g)  4NO(g) + 6H20(g) 4NO(g) + 6H20(g)
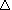 H = -906 kJ H = -906 kJ |
(1) |
The reaction is quite exothermic. In the commercial reaction, the catalyst used
in a platinum-rhodium metal gauze that is heated to about 900 oC.
However, even a hot copper wire can catalyze the reaction in the laboratory. Once
the reaction has started, the energy it produces is enough to keep the catalyst hot enough
to sustain the reaction. |
 |
|
 |
|
In the next step, the NO reacts with oxygen to produce NO2.
No catalyst is required for this reaction, as it will occur in air at room temperature.
2NO(g) + O2(g)  2NO2(g) 2NO2(g) |
(2) |
The NO2 can be compressed and cooled which will make it dimerize into N2O4,
which can then be used as an oxidizer for rocket fuel.
2NO2(g) 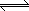 N2O4(g) + 58 kJ N2O4(g) + 58 kJ |
(3) |
|
 |
|
| From le Châtelier's principle we can predict that at high
pressures the equilibrium will be shifted to the right, since there are less molecules of
gas in the products. Similarly, at lower temperatures the reaction will shift to the
right. |
|
Instead of storing the NO2, we can use it to
produce nitric acid. The NO2(g) reacts with water to produce nitric acid
(HNO3) and NO. The nitric acid is separated by distillation, and the NO
can be recycled through reaction (2).
3NO2(g) + H2O(l)  2HNO3(aq) + NO(g) 2HNO3(aq) + NO(g) |
(4) |
|
|
|
|
The nitric acid can then be used in the manufacture of countless numbers of different
nitrogen containing compounds. For example, ammonia will react with the nitric acid
to produce ammonium nitrate, one of the most important forms of nitrogen fertilizers.
3NH3(g) + HNO3(l)  2NH4NO3(s) 2NH4NO3(s) |
(5) |


