Water Softening
Water is sometimes called "the universal solvent" because it can dissolve so
many things. As surface or underground waters pass over rocks and minerals in the
soil some of the ions in the
solids dissolve into the water. Water that contains magnesium (Mg2+) and
calcium (Ca2+) ions (and also iron or manganese ions) is known as hard water.
These ions combine with soap to create a "scum" that does not readily
dissolve. This scum – the notorious "bath tub ring" – prevents
soap from working well. The net result is the requirement to use more soap, and
clothes or skin that do not wash as cleanly.
 |
Soap is a molecule that contains a hydrophilic or
water-loving end (usually an Na+ ion), and a oleophilic or oil-loving end (an
organic fatty acid). The hydrophilic end attaches to water molecules, while the
oleophilic end dissolves into fats and oils. Thus, the soap molecules make oils
dissolve in water. |
 |
However, if the water contains a lot of ions like Mg2+ or Ca2+,
then these ions can replace the Na+ ion in the soap. These molecules
don't have nearly as strong an attraction to water, so form an insoluble scum. |
If ion
exchange resins are used to replace the hardness causing Mg2+ or Ca2+
ions, with Na+ ions, soap will do a better job of cleaning. This is an
example of an equilibrium at work.
| From le Chatelier's principle we can predict how the equilibrium will respond to the
stress. Increasing the [Na+] ions will cause the equilibrium to respond
by trying to decrease their concentration (shown in red). Then, everything on the
same side of the equation will respond in the same way, while the other side does the
opposite. |
| Applied Stress |
Le Chatelier's Principle Prediction
of Response to Stress |
|
Na+{Resin} |
+ 2 Ca2+ |
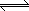 |
Ca2+{Resin} |
+ 2 Na+ |
| Increase [Na+] |
Increase |
Increase |
|
Decrease |
Decrease |
|
The process of washing the resin with brine must be done every few days, depending on
the concentration of ions in the hard water (harder water will deplete the resin more
quickly). In homes, it is usually done at night, since there is little demand for
water at this time.
Drinking softened water will not cause you to lose minerals (our food is a much more
important source of minerals than is our water). However, because softened water
contains the Na+ ions that have been displaced from the ion exchange resin, the
water does have a different taste, and should not be drunk by persons with high blood
pressure.





