|
|
|
Phenolphthalein is an organic compound (C20H14O4) used as an acid-base indicator. The compound is colorless in acidic solution and pinkish in basic solution (with the transition occuring around pH 9).
Phenolphthalein does not dissolve very well in water, so for titrations it is usually prepared in alcohol solution. When adding a drop of indicator to an acid you will sometimes detect a slight cloudy white color. This is actually a precipitate of solid phenolphthalein, as the high local concentration exceeds the solubility product. It will usually dissappear if you shake the solution, since enough solvent becomes available to dissolve the solute.
Phenolpthalein was used for many years as the active ingredient in Ex-Lax. However, recent concerns about its possible carcinogenicity have caused it to be replaced with other substances in laxatives. There is no health hazard from the minute quantities used in titrations.
The color change in phenolpthalein is due to a change in
structure of the molecule. In acid, the molecule is in its HIn form. This
structure contains a central 5 membered ring which is somewhat strained. In base the
In- structure opens up and becomes flatter.
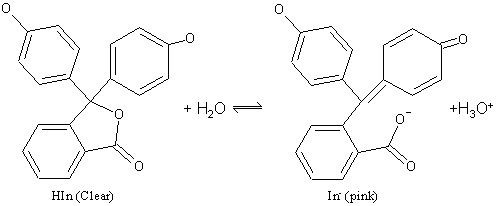
This allows the electrons more freedom, and the molecule's absorption spectrum now transmits red light. Thus there is a change in color caused by the change in molecular shape.