|
|
 Thin clouds hang over Olympus Mons, the largest known
volcano in the solar system. 25 km high, it is more than three times higher than Mt.
Everest, and at 550 km across is as wide as Saskatchewan. This photograph of the
colossal mountain, with clouds of frozen CO2, shows that Mars truly does have
an atmosphere.
(Photo courtesy: Malin Space Science Systems/NASA) |
Synthesizing Fuel: return from Mars
You should now understand equilibrium, and how to predict the effects caused by changes
in temperature or pressure using le Châtelier's principle. You can use it to make
some sense of the reactions proposed to synthesize fuel from the Martian atmosphere.
Recall that the Sabatier methanation reaction is the starting point for this process:
CO2 (g) + 4 H2 (g)  CH4 (g) + 2 H2O (g)
CH4 (g) + 2 H2O (g)
The CO2 (g) required for the reaction is abundant in the Martian
atmosphere. Even though the atmospheric pressure on Mars is very low – only 0.7
kPa, less than one hundredth of Earth's – almost 95% of the atmosphere is carbon
dioxide. Despite its thin atmosphere, this is almost 20 times as much CO2
(g) as on Earth. There will be no shortage of this resource!
H2 is a different story altogether. Until we find water on Mars, we
will have to import the hydrogen from Earth. Hydrogen is very light, but the fact
that it boils at -253 °C means there will be large evaporation losses unless it is
incredibly well insulated.
| In the Sabatier reactor, Martian CO2 will be reacted with H2
brought from Earth. Using le Châtelier's principle, predict the pressure conditions
that will give maximum yield of products.
|
The Sabatier methanation reaction takes place rapidly at a temperature of about 400 ºC
in the presence of a nickel catalyst. Which of the following statements is correct?
In order to be able to predict the effect of temperature on this reaction, you need to
know its 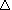 H. This can be calculated from a table of thermodynamic
values using Hess' law.
What is the calculated
H. This can be calculated from a table of thermodynamic
values using Hess' law.
What is the calculated 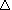 H for the reaction?
H for the reaction?
Since the Sabatier reaction is exothermic, with a 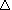 H = -165 kJ, which of the following is the correct way to write the equation?
H = -165 kJ, which of the following is the correct way to write the equation?
Complete a le Châtelier's stress table for the reaction
CO2 (g) + 4 H2 (g)  CH4 (g) + 2 H2O (g) + 165 kJ
CH4 (g) + 2 H2O (g) + 165 kJ
and apply stresses of higher and lower temperature. Based on your analysis, would
the reaction produce a greater quantity of CH4 and H2O at:
So, like the Haber process, there will be more products at
equilibrium if the temperature is low and the pressure is high. Once again we have
an example of a reaction where the kinetics – how fast we will get the products
– requires a high temperature. However, a high temperature works against the
equilibrium formation of products. A temperature of 400 ºC seems a good
choice. The experimentally determined rate is then high enough to get conversion to
products in a reasonable time, without forcing the equilibrium too far to the left.
Higher temperatures would result in a faster reaction, but we'd get less products.
The methane produced can be compressed, liquefied and stored for use as a fuel.
Some of the water would be used for human consumption, but most of it would be used to
produce oxygen gas, in the next step in the process.
