Steam Reforming of Natural Gas
 Large quantities of hydrogen gas are required in the
petrochemical industry. This hydrogen is used to change the chemical structure of
crude oil. Crude
oil is a mixture of many different molecules. Some are very light and will have
low boiling points. Others are very large, and have much higher boiling points. The
mixture can be separated by distillation; however, if you do so you will get a lot of
rather useless heavy tar, and not nearly as much of the light hydrocarbon fuel (gasoline)
as you want.
Large quantities of hydrogen gas are required in the
petrochemical industry. This hydrogen is used to change the chemical structure of
crude oil. Crude
oil is a mixture of many different molecules. Some are very light and will have
low boiling points. Others are very large, and have much higher boiling points. The
mixture can be separated by distillation; however, if you do so you will get a lot of
rather useless heavy tar, and not nearly as much of the light hydrocarbon fuel (gasoline)
as you want.
Rather than just distilling the oil to separate it into its components, modern refining
of oil relies extensively on:
- cracking: breaking down of the large hydrocarbon molecules in crude oil into smaller
ones.
- reforming: adding the broken down molecules back together in order to make the compounds
wanted.
As part of the cracking-reforming process, large quantities of hydrogen are used to
modify the structure of the cracked molecules, and reform them into the molecules wanted.
The hydrogen is usually generated from steam reforming, which is basically the
following reaction:
H2O (g) + CH4 (g) 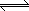 3H2
(g) + CO (g)
3H2
(g) + CO (g)
However, the reaction isn't nearly as simple to carry out as it would
appear in the above reaction.
the reaction is highly endothermic (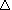 H = +206.2 kJ/mol CH4) so you have to
supply a lot of energy. This is done by burning about 25% of the methane to provide
the necessary heat: CH4 (g) + 2O2 (g)
H = +206.2 kJ/mol CH4) so you have to
supply a lot of energy. This is done by burning about 25% of the methane to provide
the necessary heat: CH4 (g) + 2O2 (g)  CO2 (g)
+ 2 H2O (g), and a catalyst is also required to get a rapid reaction.
CO2 (g)
+ 2 H2O (g), and a catalyst is also required to get a rapid reaction.
you can't burn the methane in the above reaction without using oxygen,
which will probably come from the air, and so be about 75% nitrogen. The nitrogen
will partially react to make NOx (a mixture of nitrogen oxides which are air
pollutants, and contribute to both photochemical smog and acid rain).
methane usually contains some sulfur compounds. Sulfur is a
catalytic "poison", and as well burning it would create noxious sulfur
oxides. The sulfur compounds must be stripped from the methane before it is burned.
the reaction is an equilibrium, so the reaction won't go to completion.
The water gas shift reaction: H2O (g) + CO (g) 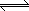 H2
(g) + CO2 (g) is used in a second stage to increase the amount of hydrogen
produced.
H2
(g) + CO2 (g) is used in a second stage to increase the amount of hydrogen
produced.
Nevertheless, complex as the process is, it can produce H2 (g)
that is more than 99.9% pure for use in further refining processes, or for the production
of other products such as ammonia.

About one-quarter of the incoming natural gas is burned to provide the
necessary energy for the reaction, while the rest is stripped of its sulfur content.
High pressure steam is added, which reacts with the methane over a nickel-alumina
catalyst. The synthesis gas contains a mixture of H2, CO2, CO,
as well as unreacted CH4 and H2O. This gas is passed into the
cooler shift reactor. The output of the shift reactor is about three quarters
hydrogen. In the presure surge adsorption unit, the impurities are removed, and
recyled back through the burner, giving more than 99.9% pure hydrogen. |
Hydrogen Fuel Cells
|
|
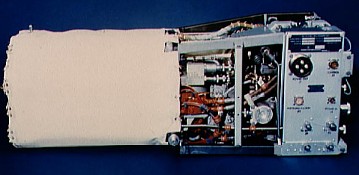 Shuttle Orbiter fuel cell (courtesy NASA) |
Hydrogen
fuel cells are potentially very pollution free and are proposed as a method of
powering automobiles. In them, H2 and O2 gases react to form
water and release energy – not through combustion, but through electrochemical
processes.
H2 (g) + O2 (g)  H2O (g) H2O (g) |
|
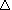 H = -241.8 kJ H = -241.8 kJ |
|
(1) |
This reaction produces more energy per unit mass than any other chemical
reaction. However, that is not the reason it would be used to power an
automobile. Since the only product of the reaction is water, the reaction is
pollution free. Fuel cell technology is well developed because of its use in the
space program. The potential for clean, noise free automobiles is enticing.
The problem is that H2 cannot be considered a fuel. There
is virtually no free hydrogen on earth. It is almost all combined into water, and
hydrocarbons. Thus in order to make use of the fuel cell we have to have a way of
producing the hydrogen gas. Steam reforming is an obvious potential candidate.
Suppose that the CH4 were simply burned in an internal
combustion engine. Then, if we had 100% combustion, and no energy loss through
friction, and other heat losses, we would get:
CH4 (g) + 2O2 (g)  CO2 (g) + 2H2O (g) CO2 (g) + 2H2O (g) |
|
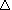 H = -802.2 kJ H = -802.2 kJ |
|
(2) |
However, an internal combustion engine is actually only about 20%
efficient, so we probably get closer to 160 kJ/mol CH4 burned to
move the car. In addition we still get all the noxious NOx (nitrogen
oxides) which contribute to photochemical smog and acid rain air pollution, since these
products are always formed in high temperature combustion reactions.
Now let's examine what happens if we reform the CH4 (g) into
hydrogen.
H2O (g) + CH4 (g)  3H2 (g) + CO (g) 3H2 (g) + CO (g) |
|
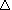 H = +206.2 kJ H = +206.2 kJ |
|
(3) |
H2O (g) + CO (g)  H2 (g) + CO2 (g) H2 (g) + CO2 (g) |
|
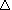 H = -41.2 kJ H = -41.2 kJ |
|
(4) |
which gives an overall reaction of
2H2O (g) + CH4 (g)  4H2 (g) + CO2 (g) 4H2 (g) + CO2 (g) |
|
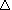 H = +165.0 kJ H = +165.0 kJ |
|
(5) |
Since the overall reaction produces 4 mol H2 (g), if all this hydrogen were
combined in the fuel cell, we would get a net energy production of:
2H2O (g) + CH4 (g)  4H2 (g) + CO2 (g) 4H2 (g) + CO2 (g) |
|
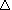 H = +165.0 kJ H = +165.0 kJ |
|
(5) |
4H2 (g) + 4O2 (g)  4H2O (g) 4H2O (g) |
|
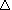 H = -967.2 kJ H = -967.2 kJ |
|
(6) |
which, adding (5) and (6) gives an overall reaction of
CH4 (g) + 2O2 (g)  CO2 (g) + 2H2O (g) CO2 (g) + 2H2O (g) |
|
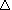 H = -802.2 kJ H = -802.2 kJ |
|
(7) |
Equations (2) and (7) are exactly the same. You get the same amount of energy as
if you just burned the CH4 to begin with! Actually this shouldn't be
surprising – it is just the law of conservation of energy at work. So why
bother going the fuel cell route?
|
|
 Hydrogen fuel cell powered bus
(photo courtesy NASA) |
There are at least five reasons:
- fuel cells are a lot more efficient than internal combustion engines. Fuel cells
can get up to 75% efficiency. We have to burn about 25% of the CH4 to
provide the energy for the reforming reaction, but that still leaves us 75% of the 802.2
kJ or about 600 kJ. If 75% of this can then be converted into useable work in a fuel
cell, we will get about 450 kJ of useful work. Compare that to the perhaps 160 kJ
from burning the fuel directly. Your car will be able to travel two to three times
as far as on the same amount of hydrocarbon fuel.
- an efficient catalyst can lower the temperature required. The lower the
temperature the less NOx will be produced when we burn the CH4 to
provide the energy required for reforming. This causes less nitrogen oxide air
pollution.
- we still produce the same amount of CO2 per mole of CH4
used. But, since we can go much farther on the same amount of fuel, the net
contribution to global warming causing pollution is much less.
- fuel cells raise the potential of using reuseable biomass which can be efficiently and
cheaply converted into liquid fuels like alcohols. Reusing biomass would not
increase the global CO2 balance.
- electric motors are quiet.
However, hydrogen is a very difficult substance to store. Active research is
ongoing to try and develop small catalytic reformers that can convert hydrocarbons, or
alcohols directly into hydrogen. These reformers would be in the car itself,
allowing the car to use easily stored liquid fuels, much as they do today. Even more
exciting is the potential for direct conversion of liquid alcohol fuels without the need
for a reformer; however, such fuel cells are still very experimental.
 Large quantities of hydrogen gas are required in the
petrochemical industry. This hydrogen is used to change the chemical structure of
crude oil. Crude
oil is a mixture of many different molecules. Some are very light and will have
low boiling points. Others are very large, and have much higher boiling points. The
mixture can be separated by distillation; however, if you do so you will get a lot of
rather useless heavy tar, and not nearly as much of the light hydrocarbon fuel (gasoline)
as you want.
Large quantities of hydrogen gas are required in the
petrochemical industry. This hydrogen is used to change the chemical structure of
crude oil. Crude
oil is a mixture of many different molecules. Some are very light and will have
low boiling points. Others are very large, and have much higher boiling points. The
mixture can be separated by distillation; however, if you do so you will get a lot of
rather useless heavy tar, and not nearly as much of the light hydrocarbon fuel (gasoline)
as you want.

