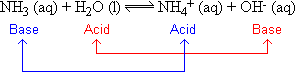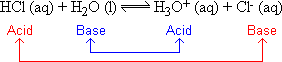
Bar: the standard unit of atmospheric pressure has been defined as 1 bar, which is equal to 100 kPa. In older texts you will see reference to the standard as 1 atm. There is a very slight difference between the new and old standard: 1 atm = 1.01325 bar.
Boiling point: the temperature at which a liquid's vapor pressure is the same as the externally applied pressure on the liquid. For water, the normal boiling point at sea level is 100 oC.
Boyle's Law: one of the original pieces of evidence which led to our understanding of matter was the statement of Robert Boyle (1627-1691) about the effect of changing the pressure on the volume of a gas. In "A Defence of the Doctrine Touching the Spring And Weight of the Air" published in 1662, Boyle shows data that clearly illustrates that there is an inverse relationship between the pressure of a gas and its volume. In other words, if the pressure doubles, then volume becomes half as large.
This is often stated mathematically as: P1V1 = P2V2
where P1 is the pressure at a certain volume V1 while P2 is the pressure the gas will have if the volume is changed to V2.
In continental Europe, Boyle's law is sometimes called Mariotte's Law
Brönsted-Lowry definition: in this definition of an acid or base:
For example, HCl can be considered to be an acid since it can donate a proton to water in the following reaction:
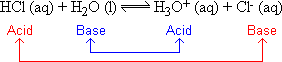
Note that HCl, the acid, donates a proton, H+, to the base which is water. In this definition acids and bases always go together in pairs. The acid on one side of the equilibrium sign is paired with a base on the other. These pairs that go together are called "conjugate" acid-base pairs. In the above example HCl the acid is paired with Cl- the base. Similarly, H2O the base is paired with its conjugate acid, H3O+.
NH3 acts as a base when it is mixed with water, since it can accept a proton from water acting as a base: