So what is the unit for atomic mass?
The answer is simple - there isn't one! Atomic masses are relative masses. This means
that they are unitless.
The original idea of relative atomic masses came from John Dalton. Others before
Dalton - Lavoisier for one - had used the concept of combination of simple substances into
more complex compounds. However, it was Dalton who realized that if these
particles had their own unique mass they could account for the Law of Conservation of
Mass.
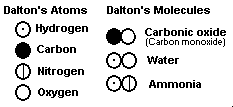 Dalton believed, mistakenly,
that only one atom of one kind could combine with another. He invented a series of
symbols to represent each atom. Notice that only his formula for carbon monoxide
would be considered correct today. He analyzed the decomposition of water, which
produces eight weights of oxygen for every one weight of hydrogen, and concluded
(incorrectly, since he was using the wrong formula for water) that an oxygen
"simple", an atom, was eight times heavier than a hydrogen atom. Though
his idea of atoms was brilliant, his mistaken concept that compounds had only single atoms
of each element caused him to reject many ideas that later turned out to be much better -
including that of Avogadro. Nevertheless Dalton's concept
of relative mass was doubtless his greatest contribution to chemistry. Dalton believed, mistakenly,
that only one atom of one kind could combine with another. He invented a series of
symbols to represent each atom. Notice that only his formula for carbon monoxide
would be considered correct today. He analyzed the decomposition of water, which
produces eight weights of oxygen for every one weight of hydrogen, and concluded
(incorrectly, since he was using the wrong formula for water) that an oxygen
"simple", an atom, was eight times heavier than a hydrogen atom. Though
his idea of atoms was brilliant, his mistaken concept that compounds had only single atoms
of each element caused him to reject many ideas that later turned out to be much better -
including that of Avogadro. Nevertheless Dalton's concept
of relative mass was doubtless his greatest contribution to chemistry.
| Which of the following are correct? Check all that apply.
|
Why are atomic masses unitless? It is because they are relative masses
where the mass of one atom is compared to the other. Although we can't find the
mass of any individual atom, we can easily find the mass of a bunch. If we could
find the mass of the same sized bunch of atoms of two different kinds, say Hydrogen
and Oxygen, then we could compare their masses. Provided that we have the same number
of atoms in each bunch, this is easy to do. This is where the work of Avogadro becomes so important.
A million is a bunch. However, it is not a very big bunch as you can see from
these numbers:
| mass of 1 million oxygen molecules |
53.16 x 10-18 g |
| mass of 1 million hydrogen molecules |
3.349 x 10-18 g |
|
Copyright © 1998 - 2008 David
Dice
|
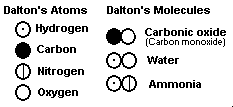 Dalton believed, mistakenly,
that only one atom of one kind could combine with another. He invented a series of
symbols to represent each atom. Notice that only his formula for carbon monoxide
would be considered correct today. He analyzed the decomposition of water, which
produces eight weights of oxygen for every one weight of hydrogen, and concluded
(incorrectly, since he was using the wrong formula for water) that an oxygen
"simple", an atom, was eight times heavier than a hydrogen atom. Though
his idea of atoms was brilliant, his mistaken concept that compounds had only single atoms
of each element caused him to reject many ideas that later turned out to be much better -
including that of
Dalton believed, mistakenly,
that only one atom of one kind could combine with another. He invented a series of
symbols to represent each atom. Notice that only his formula for carbon monoxide
would be considered correct today. He analyzed the decomposition of water, which
produces eight weights of oxygen for every one weight of hydrogen, and concluded
(incorrectly, since he was using the wrong formula for water) that an oxygen
"simple", an atom, was eight times heavier than a hydrogen atom. Though
his idea of atoms was brilliant, his mistaken concept that compounds had only single atoms
of each element caused him to reject many ideas that later turned out to be much better -
including that of