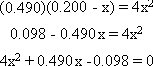Calculating K – More Examples
All the examples to this point have been relatively easy because we knew the
concentration of all but one substance in the K expression, at equilibrium.
In the examples on this page we will be missing some of the concentrations at equilibrium,
but each problem will have enough information to get to that point, so that you can then
use Keq to get the missing values.
To do this, we need to make use of the balanced chemical equation, and what we know
about the coefficients in the reaction. Remember that the coefficients tell us the ratio in which the reactants react, and the products
are formed, but not how much there is of any substance at equilibrium (nor at the
start of the reaction). In general we always have, or can figure out, three pieces
of information about any chemical reaction:
- the concentrations of the reactants and/or products when the reaction started (initial
values)
- how the concentrations change because of the chemical reaction (reaction change)
- the concentrations of each reactant and product when the reaction is finished
(equilibrium values)
In previous problems where we had to calculate Keq we made use of an IRE
table. We can often use the same kind of table when we already know Keq.
| Example 1: 0.100 mol of I2 and 0.100
mol of H2 gases are placed in a 1.00 L flask and heated to 400 șC. At
this temperature Kc = 36.0. Hydrogen and iodine gases react to from
hydrogen iodide. After equilibrium has been reached what will the concentrations of
all the products and reactants be? Step 1: Write out the Kc expression for
the balanced equation.
From the way the problem is stated, this reaction is I2 (g) + H2
(g) 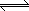 2HI (g)
for which 2HI (g)
for which 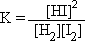 =
36 =
36
| Step 2: Set up an IRE table, and complete the parts we know. We are
given starting quantities of hydrogen and iodine. Because of the way the problem is
defined, there was no hydrogen iodide in the container initially. |

 Click
here for a step by step
explanation of how to complete the IRE table to solve this problem. Click
here for a step by step
explanation of how to complete the IRE table to solve this problem.
|
Step 3: Substitute these values for equilibrium into the K expression and do the math
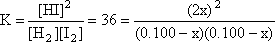
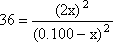
This is a quadratic equation. It might look difficult, but
fortunately it is an example of the type of quadratics known as "perfect
squares". This actually makes it easy to solve. Just take the square
root of both side.
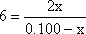
Then "cross multiply" and collect the x terms together on one
side
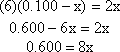
Finally, solve for x
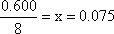
We are now close to the final answer. We need to substitute x for
each value in the Equilibrium row of the table, to get the final answers.
| [H2] |
= 0.100 - x |
= 0.100 - 0.075 |
= 0.025 mol/L |
| [I2] |
= 0.100 - x |
= 0.100 - 0.075 |
= 0.025 mol/L |
| [HI] |
= 2x |
= 2(0.075) |
= 0.150 mol/L |
|
Example 2: 0.200 mol of N2O4
(g) is put in a 1.00 L flask and comes to equilibrium at 100 șC where Kc =
0.490 for the reaction N2O4 (g) 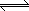 2NO2 (g). What will the
equilibrium concentrations of N2O4 (g) and NO2 (g) be? 2NO2 (g). What will the
equilibrium concentrations of N2O4 (g) and NO2 (g) be?Step
1: Write out the K expression for the balanced equation: 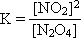 = 0.490 = 0.490
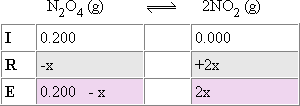
| Step 2: Identify each concentration at equilibrium. To do this set
up and complete the IRE table. |
 Click
here for a step by step
explanation of how to use an IRE table to solve this problem. Click
here for a step by step
explanation of how to use an IRE table to solve this problem.
|
|
|

Click to see an alternative method (iterative solution) for the math in this type of
problem.
|
Step 3: Substitute these values for equilibrium into K and do the math
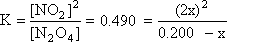
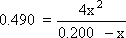
Once again, this is a quadratic equation. However, this time there
is no simple solution. Change the equation into a quadratic equation in standard
form.
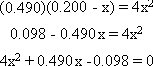
Now, you can solve it using the quadratic formula 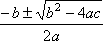
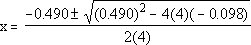
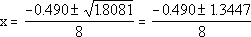
| The two possible answers for x are then: |
|
x = -0.229 |
| x = +0.107 |
| which give possible answers for the reactants and products |
|
[N2O4] |
[NO2] |
| x = -0.229 |
= 0.200 - x
= 0.200 - (-0.229)
= 0.200 + 0.229
= 0.429
|
= 2x
= 2(-0.229)
= -0.458 |
| x = +0.107 |
= 0.200 - x
= 0.200 - (+0.107)
= 0.200 - 0.107
= 0.093 |
= 2x
= 2(+0.107)
= 0.214 |
Since the negative value of x would result in a negative value of NO2
it is not meaningful. Therefore the positive value is the correct answer, and the
concentrations of N2O4 = 0.093 mol/L, while the
concentration of NO2 is 0.214 mol/L. |
Summary:
| Use an IRE table whenever you don't know all the concentrations at equilibrium. |
 Try the following sample problems
to check your understanding.
Try the following sample problems
to check your understanding.
![]() 2HI (g)
for which
2HI (g)
for which ![]() =
36
=
36![]()
![]()
![]()
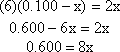
![]()