1. 0.100 mol of NO2 (g) is added to an evaculated 1 L container. K
for the reaction 2NO2 (g) 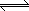 N2O4 (g) is 170. What is the equilibrium
concentration of N2O4 and NO2? N2O4 (g) is 170. What is the equilibrium
concentration of N2O4 and NO2? |
 Click to work out the problem and check your answer. Click to work out the problem and check your answer.
|
|
| 2. 4.00 x 10-2 mol of COCl2 (phosgene) is placed in a 1.0 L
container and heated to 500K. At this temperature K = 8.5 x 10-5 for the
reaction COCl2 (g) 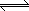 CO (g) + Cl2 (g) CO (g) + Cl2 (g)
What is the equilibrium concentration of CO (g)? |
 Click to work out
the problem and check your answer. Click to work out
the problem and check your answer.
|
|
| 3. 0.20 mol of NOCl was initially placed in a 2.0 L container at 280 șC. K
= 2.70 x 10-3 for the reaction 2NOCl (g) 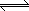 2NO (g) + Cl2 (g) 2NO (g) + Cl2 (g)
What is the equilibrium concentration of NO? |
 Click to work out
the problem and check your answer. Click to work out
the problem and check your answer.
|
|
| 4. Steam reforming of methane is used extensively in the
petroleum industry to generate hydrogen gas. Suppose that equal amounts of H2O
and CH4 gases are fed into the reactor. The reaction is in
equilibrium H2O (g) + CH4 (g) 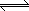 3H2 (g) + CO (g) 3H2 (g) + CO (g)
at 1000 șC where K = 0.800. In the products there are equal
concentrations of water and methane of 0.280 mol/L. What is the concentration of H2
and CO gases? |
 Click to work out the problem and check your answer. Click to work out the problem and check your answer.
|
![]() Click to work out the problem and check your answer.
Click to work out the problem and check your answer.![]() CO (g) + Cl2 (g)
CO (g) + Cl2 (g)![]() Click to work out
the problem and check your answer.
Click to work out
the problem and check your answer.![]() 2NO (g) + Cl2 (g)
2NO (g) + Cl2 (g)![]() Click to work out
the problem and check your answer.
Click to work out
the problem and check your answer.![]() 3H2 (g) + CO (g)
3H2 (g) + CO (g)![]() Click to work out the problem and check your answer.
Click to work out the problem and check your answer.