Calculations When the Value of K is Known
Most of the time, you actually will know the equilibrium constant for a reaction, since
someone else has already measured it. This means that you can calculate the amount
of products you expect a certain reaction to give, without having to do the reaction at
all.
This is an incredibly useful thing to be able to do. If you are trying to design
a Sabatier reactor to generate methane fuel from
the Maratian atmosphere, you want to know quantitatively the kinds of conditions that will cause high product
yield before you start designing the system. Using known values of Keq
for the reaction you can predict whether it is even feasible to build such a system.
Spending millions of dollars to build a system that will hardly make any useful product is
hardly sensible. You need to check out the conditions before you start the design.
With the value of Keq, the balanced equation, and some of the concentrations
you can work out the calculation of each substance at equilibrium.
Example 1: K for the reaction H2 (g) + I2
(g) 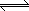 2HI (g)
at 450 șC is 22.5. If at equilibrium, the [H2] = 3.4 x 10-2
mol/L, [I2] = 9.0 x 10-3 mol/L, then what
is the [HI]? 2HI (g)
at 450 șC is 22.5. If at equilibrium, the [H2] = 3.4 x 10-2
mol/L, [I2] = 9.0 x 10-3 mol/L, then what
is the [HI]?This is an easy problem to solve because there is only one unknown –
the concentration of [HI]. We will represent this unknown by the algebraic variable
x.
Step 1: Write out the K expression for the balanced equation 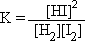 = 22.5 = 22.5
Step 2: Let the unknown concentration of HI be x. Substitute this and the known
concentrations into the K expression
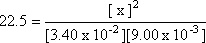
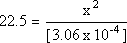
Step 3: Isolate the unknown value.
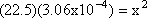
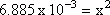
Step 4: Solve for x (in this case take the square root of both sides)
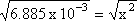
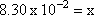
Step 5: Substitute x back for its actual value
[HI] = x = 8.30 x 10-2 |
Example 2:At 4000 oC Keq = 3.94 x
102 for the reaction 2H2 (g) + O2 (g) 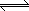 2H2O
(g). If at equilibrium, the [H2] = 1.60 x 10-2 mol/L,
and [H2O] = 3.02 x 10-2 mol/L, then what is the [O2]? 2H2O
(g). If at equilibrium, the [H2] = 1.60 x 10-2 mol/L,
and [H2O] = 3.02 x 10-2 mol/L, then what is the [O2]?There
is only one unknown – the concentration of [O2]. We will represent
this unknown by the algebraic variable x.
Step 1: Write out the K expression for the balanced equation 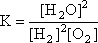 = 3.94 x 102 = 3.94 x 102
Step 2: Let the unknown concentration of O2 be x. Substitute this
and the known concentrations into the K expression
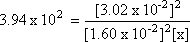
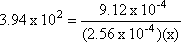
Step 3: Isolate the unknown value.
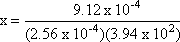
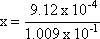
Step 4: Solve for x.
x = 9.04 x 10-3
Step 5: Substitute x back for its actual value
[O2] = x = 9.04 x 10-3 |
Try the following problems:
| To enter numbers in scientific notation on the
computer, use "E" notation. For example: 2.34 x 105 is 2.34E5,
2.34 x 10-5 is 2.34E-5 |
Click here for more example problems.
 Measuring K for the solubility of calcium hydroxide
Measuring K for the solubility of calcium hydroxide
![]() = 22.5
= 22.5![]()
![]()
![]()
![]()
![]()
![]()