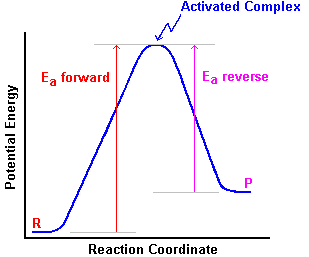
Activated complex: a highly unstable molecule in transition between reactants and products, at the peak of the activation energy diagram. It will fall apart on its first vibration into either reactant molecules, or product.
Activation energy: the minimum amount of energy required for a reaction to occur. In general, the activation energy for the forward and reverse reactions are different. Graphically, activation energy is often illustrated as:
 |
The activation energy (Ea) for the forward reaction is the potential energy difference between the activated complex and the reactants. For the reverse reaction it is the potential energy difference between the activated complex and the products. |
Activity: a quantity that expresses the reactivity of a substance in a chemical reaction. In real systems there are often substances present that affect the reaction, but are not a part of the reaction itself. This is especially true in ionic solutions, since the presence of the positive and negative ions can have a substantial polarity effect on the solution. Thus these substances can affect the equilibrium constant. In an "ideal" solution, such effects are not present, and then the activity of a substance equals its concentration. Real solutions approach ideal behavior when they are dilute. In beginning chemistry it is generally assumed that the concentration and activity are the same, though this is often not true.
Atmospheric Pressure: the pressure exerted by the earth's air. At the Earth's surface the pressure is about 101.3 kPa. Atmospheric pressure decreases with altitude, since there is less mass of air above a given point as the altitude increases. The approximate pressure of the earth's atmosphere can be calculated from the formula:
| P | = | (Psurface)(e-h/H) |
| where | ||
| P | = | pressure at a given altitude h |
| Psurface | = | pressure at the earth's surface |
| e | = | Euler's number |
| h | = | desired altitude (km) |
| H | = | scale height of atmosphere (about 7 km) |
You can use the exponentiation key (ex) on a calculator to solve this equation
.