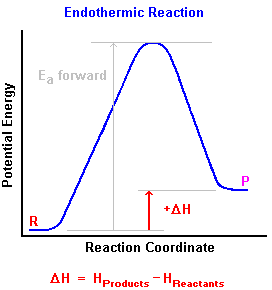
On this potential energy graph DH is represented by the vertical distance from reactants to products. This shows an increase in energy of the system, so the forward reaction is endothermic.
Endothermic reactions have a positive DH.
Empirical: based on observation. All theoretical explanations in science must be backed up by empirical evidence.
| Endothermic: reacting systems which absorb heat from the surroundings. Heat "goes into" the system,
so DH is positive. The opposite
of exothermic. On this potential energy graph DH is represented by the vertical distance from reactants to products. This shows an increase in energy of the system, so the forward reaction is endothermic. Endothermic reactions have a positive DH. |
 |
Endpoint: the point in a titration where there is a distinct and measureable change. When using a colored indicator, as in an acid-base titration, it the point where the color of the indicator changes. A good indicator will change very close to the equivalence point of the reaction.
Enthalpy: also known as heat content, is the change in energy of a reaction at constant pressure. Symbolized by H, the absolute enthalpy of any state is impossible to measure. Instead we always measure changes in enthalpy, DH. The enthalpy change for a chemical change can be calculated using Hess's law.
Entropy: a measurement of the disorder or randomness of a system. Entropy is symbolized by S and entropy change by DS. The entropy of a pure crystalline substance is 0 at absolute zero, and increases as the temperature increases. The second law of thermodynamics states that "every system left to itself will change to a position of maximum entropy".
Equilibrium: a state of balance in a closed system at constant temperature, in which no macroscopic properties change, because the rate of the forward reaction has become equal to the rate of the reverse reaction.
Equilibrium constant: a numerical value represented by the letter K, mathematically equal to the concentration of all the product species, divided by the concentration of all the reactant species, each concentration raised to the power of the coefficient of that substance. For example, for an imaginary reaction:
aA + bB + cC ... ![]() ... + xX + yY + zZ
... + xX + yY + zZ
the equilibrium constant expression is ![]()
Note: although this is the way the equilibrium constant expression is usually written in beginning chemistry texts, the term concentration used above is correct only for ideal solutions, and should be replaced by the activity.
Equivalence point: the point in a titration where the same chemical quantity of substances has been added. For example, in an acid-base titration it is the point where mol H+ = mol OH-. When an indicator dye is used to detect the change, its endpoint should be close to the equivalence point.
Escape velocity: the minimum speed
which a spacecraft must attain in order to escape from the gravitational field of a planet
or star. The escape velocity is ![]() where G
is the universal gravitational constant (6.673 x 10-11 m3/kg·
s2) ; m is the mass of the planet or star (kg); and r is the
radial distance (m) from the center of the planet or star. For earth, at the surface
it is about 40,300 km/hr.
where G
is the universal gravitational constant (6.673 x 10-11 m3/kg·
s2) ; m is the mass of the planet or star (kg); and r is the
radial distance (m) from the center of the planet or star. For earth, at the surface
it is about 40,300 km/hr.
| Exothermic: reacting systems which release heat into the surroundings. Heat "exits" from the
system, so DH is negative. The
opposite of endothermic. On this potential energy graph, DH is represented by the vertical distance from reactants to products. This is a decrease in energy of the system, so the forward reaction is exothermic. Exothermic reactions have a negative DH. |
 |
Experimental error: all experiments contain errors in their measured data. Errors are of two types:
Both types of errors must be recognized, and considered when doing any experiment to ensure that the results are consistent "within experimental error."