Calciferous: animals that remove carbonate from water by combining it with calcium ions to form insoluble calcium carbonate. Typical examples are corals, molluscs, crustaceans, and some algae.
Catalyst: a substance that increases the rate of a chemical reaction, but is not itself consumed. A catalyst lowers the activation energy by adding new lower energy pathways to a reaction mechanism. The catalyst is not consumed since one of the added steps to the mechanism regenerates the catalyst.
le Châtelier's principle: (see L)
Classical mechanics: the treatment of matter without using quantum mechanics. In classical mechanics, small particles behave the same way as large particles do, though in a much scaled down manner. For example, in classical mechanics, molecules of gases can be treated as small spherical objects colliding with each other and with the walls of their container.
Closed system: a reaction system in which no matter can enter or exit . A closed system can reach an equilibrium. The opposite of an open system.
Coefficient: the numbers in front of a
chemical formula in a balanced equation which indicate the ratio of molecules which will
react. In the equation H2 (g) + I2 (g) ![]() 2 HI (g) the coeffient 1 is understood
to be in front of H2 and I2 and the coefficient in front of HI is 2.
2 HI (g) the coeffient 1 is understood
to be in front of H2 and I2 and the coefficient in front of HI is 2.
Coke: coke is almost 100% carbon, and is produced by heating bituminous (soft) coal in the absence of oxygen to over 1000 ºC. The more volatile components are driven off (coal gas) and used for the production of other materials. The major use of coke is as the source of carbon for manufacturing steel.
| Color temperature: commonly used in the photographic industry to measure the color of lighting. It is the color an object would have if it were that temperature. Incandescent lights are cooler than daylight, having color temperatures around 3000 K, and so have a reddish-orange tint. Natural daylight is much bluer having a color temperature around 5000 K. The dyes in film that react to light are color sensitive, so they respond differently to the different light. This will cause color shifts in the film. Pictures taken with an incandescent lamp on film designed for daylight will have a very orange, warm color. While not necessarily unpleasant, it is not a true color rendition | 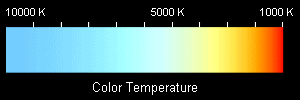 |
Common ion effect: when a solution has an ion introduced from another source that is the same as one of the ions in the original solution, the effect predicted by le Châtelier's principle is called the common ion effect. For example, if you had the following equilibrium in which potassium chromate was dissolved in water:
2K+ (aq) + 2CrO42- (aq) + 2 H+
(aq) ![]() 2K+ (aq) + Cr2O72- (aq)
+ H2O (l)
2K+ (aq) + Cr2O72- (aq)
+ H2O (l)
and added some sodium chromate, the chromate ion CrO42- is the common ion.
Complete reaction: a reaction in which almost all of at least one reactant has been used up. This will happen when the rate of the reaction in one direction is much, much greater than the rate in the opposite direction. Such reactions are rarely treated as equilibria.
Concentration: quantity of solute per unit quantity of solvent. Usually measured in molarity. Molar concentration is symbolized by square brackets [ ]. When dealing with equilibrium changes, concentration is usually used in beginning chemistry instead of the more accurate term: activity.
Critical point: the highest temperature and presure at which a substance can still be a liquid. At temperatures higher than the critical point, liquids and gases are indistinguishable, and are called supercritical fluids.